Difference Between Enzyme and Catalyst: In the intricate tapestry of biochemical reactions that govern life, enzymes and catalysts stand out as pivotal players. Both essential to speeding up chemical processes, these entities harbor nuanced distinctions. Let's embark on a journey to unravel the key difference between enzymes and catalysts:
Check out Best Scrubs for Medical Professionals Here!
Difference Between Enzyme and Catalyst
This table summarizes the key difference between enzymes and catalysts, emphasizing their distinct characteristics.
|
Feature |
Enzyme |
Catalyst |
|
Nature |
Biological macromolecule, often a protein. |
General term encompassing substances that accelerate reactions. Can be organic, inorganic, or synthetic. |
|
Specificity |
Highly specific, each designed for a particular reaction or group of reactions. |
Can be specific or non-specific, promoting a range of reactions without precision. |
|
Active Site |
Possesses well-defined active sites where substrates bind for catalysis. |
May not have well-defined active sites, relying on general interactions with reactants. |
|
Biological Context |
Primarily operates within living organisms, contributing to the regulation and coordination of biochemical pathways. |
Can function in biological systems but are not confined to living organisms; can be synthetic or inorganic. |
|
Regulation |
Subject to intricate regulation influenced by factors like substrate concentration, pH, and temperature. |
Generally lacks sophisticated regulatory mechanisms inherent in enzymatic processes. |
|
Examples |
Amylase, catalase, DNA polymerase. |
Platinum, palladium in catalytic converters, synthetic zeolite catalysts. |
|
Function |
Facilitates and accelerates specific biochemical reactions within living organisms. |
Accelerates chemical reactions but is not limited to biological systems; can be used in various industrial processes. |
What is Enzyme?
An enzyme is a biological molecule, typically a protein, that acts as a catalyst to facilitate and accelerate chemical reactions within living organisms. Enzymes are fundamental to the functioning of cells and play a crucial role in various physiological processes. They are essential for life as they enable and regulate the myriad of chemical reactions required for cellular activities.
Key characteristics of enzymes include:
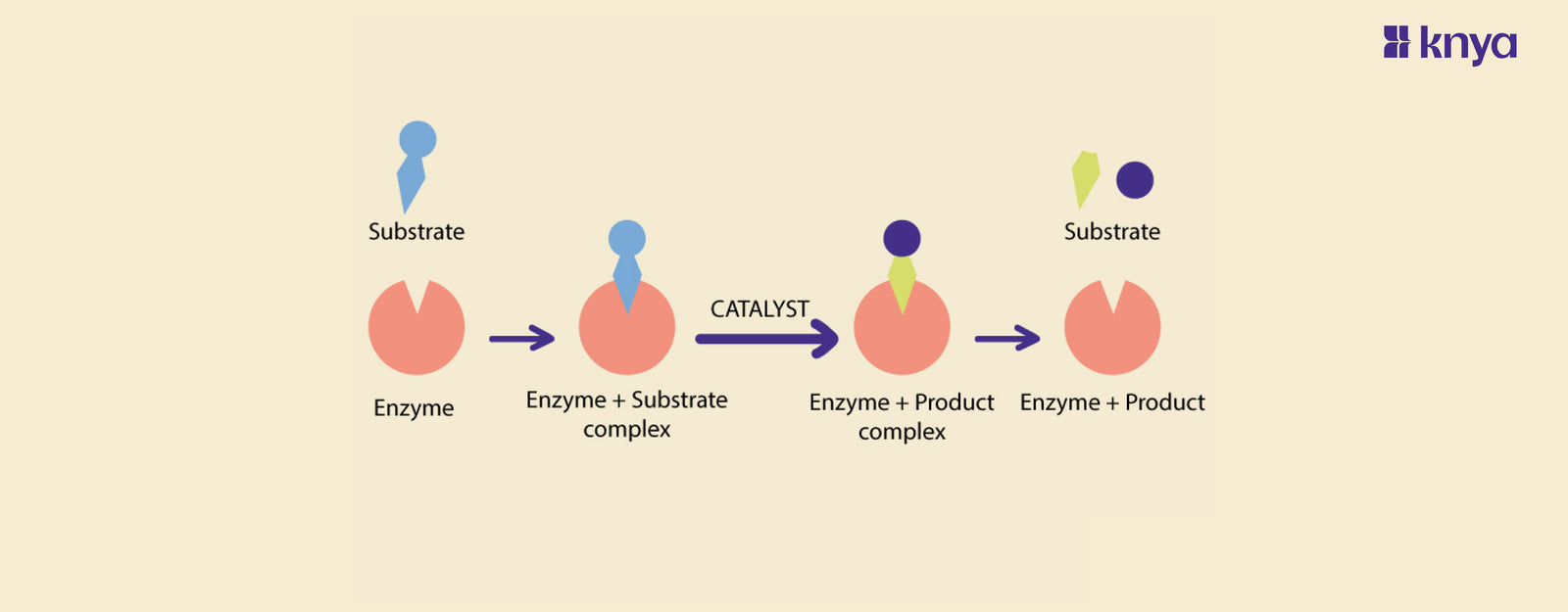
- Catalytic Activity: Enzymes speed up chemical reactions by lowering the activation energy required for the reactions to occur. They do not alter the equilibrium or final products of the reactions they catalyze.
- Specificity: Each enzyme is highly specific to a particular reaction or group of similar reactions. This specificity arises from the enzyme's unique three-dimensional structure, and it is often compared to a lock and key mechanism.
- Active Site: Enzymes have a region called the active site, where the substrate (the molecule upon which the enzyme acts) binds. The interaction between the active site and the substrate initiates the catalytic process.
- Regulation: Enzyme activity is often regulated in response to the needs of the cell or organism. Various factors, such as substrate concentration, pH, and temperature, can influence the rate of enzyme-catalyzed reactions.
- Biological Context: Enzymes are integral to the biochemical pathways that occur within living organisms. They contribute to metabolic processes, energy production, DNA replication, and various other cellular functions.
Examples of enzymes include amylase, which breaks down starch into sugars; catalase, which catalyzes the breakdown of hydrogen peroxide; and DNA polymerase, responsible for synthesizing DNA during replication.
What is a Catalyst?
A catalyst is a substance that increases the rate of a chemical reaction by providing an alternative pathway with a lower activation energy, without being consumed or permanently altered in the process. Catalysts enable reactions to occur more rapidly, making them crucial in various industrial, environmental, and biological processes.
Key features of catalysts include:
- Catalytic Activity: Catalysts function by lowering the energy barrier (activation energy) required for a reaction to proceed, facilitating the formation of products.
- Specificity: Catalysts can be specific or non-specific. Specific catalysts promote a particular reaction or group of reactions, while non-specific catalysts may enhance a range of reactions.
- Active Sites: Unlike enzymes, catalysts may not have well-defined active sites. Their interaction with reactants is generally less specific compared to enzymatic reactions.
- Regeneration: Catalysts remain unchanged at the end of a reaction and can be used repeatedly. They are not consumed in the reaction they facilitate.
- Biological and Industrial Applications: Catalysts are utilized in various fields, including industrial processes such as the production of chemicals, environmental applications, and biological reactions within living organisms.
Check out Lab Coats with Best Quality
Similarity Between Enzyme and Catalyst
There are key difference between enzyme and catalyst but Enzymes and catalysts share similarities as they both play critical roles in facilitating chemical reactions, but they differ in their nature and specificity. Here are the key similarities between enzymes and catalysts:
- Facilitation of Reactions:
- Catalysts: Catalysts, in a general sense, are substances that increase the rate of a chemical reaction without undergoing any permanent change themselves. They achieve this by providing an alternative pathway for the reaction with a lower activation energy.
- Enzymes: Enzymes, a specific type of catalyst, also enhance the rate of chemical reactions by lowering the activation energy required for the reaction to proceed. They act as biological catalysts in living organisms.
- Not Consumed in Reactions:
- Catalysts: Catalysts are not consumed in the reactions they facilitate. They remain unchanged chemically and can be used repeatedly in multiple reactions.
- Enzymes: Similarly, enzymes are not consumed during the reactions they catalyze. They can be recycled and used for multiple cycles of the same reaction.
- Specificity:
- Catalysts: Catalysts can be general or specific, depending on their nature. Some catalysts may work for a broad range of reactions, while others exhibit specificity for certain types of reactions.
- Enzymes: Enzymes are highly specific. Each enzyme typically catalyzes a particular reaction or a set of closely related reactions. This specificity is often due to the unique shape and active site of the enzyme, which matches the substrate molecules involved in the reaction.
- Effect on Reaction Equilibrium:
- Catalysts: Catalysts do not affect the equilibrium position of a reaction. They accelerate both the forward and reverse reactions, helping the system reach equilibrium more quickly.
- Enzymes: Enzymes also do not alter the equilibrium position of a reaction. They accelerate the attainment of equilibrium by facilitating the conversion of reactants to products and vice versa.
While enzymes and catalysts share the fundamental role of facilitating chemical reactions by lowering activation energy, The difference between enzyme and catalyst lie in specificity and origin. Enzymes, as biological catalysts, exhibit a remarkable specificity for particular reactions, driven by their unique structures and active sites. Catalysts, on the other hand, can be either chemical or biological, with a broader range of applicability. Both entities contribute significantly to the acceleration of reactions, allowing for the efficient and controlled progression of chemical transformations.